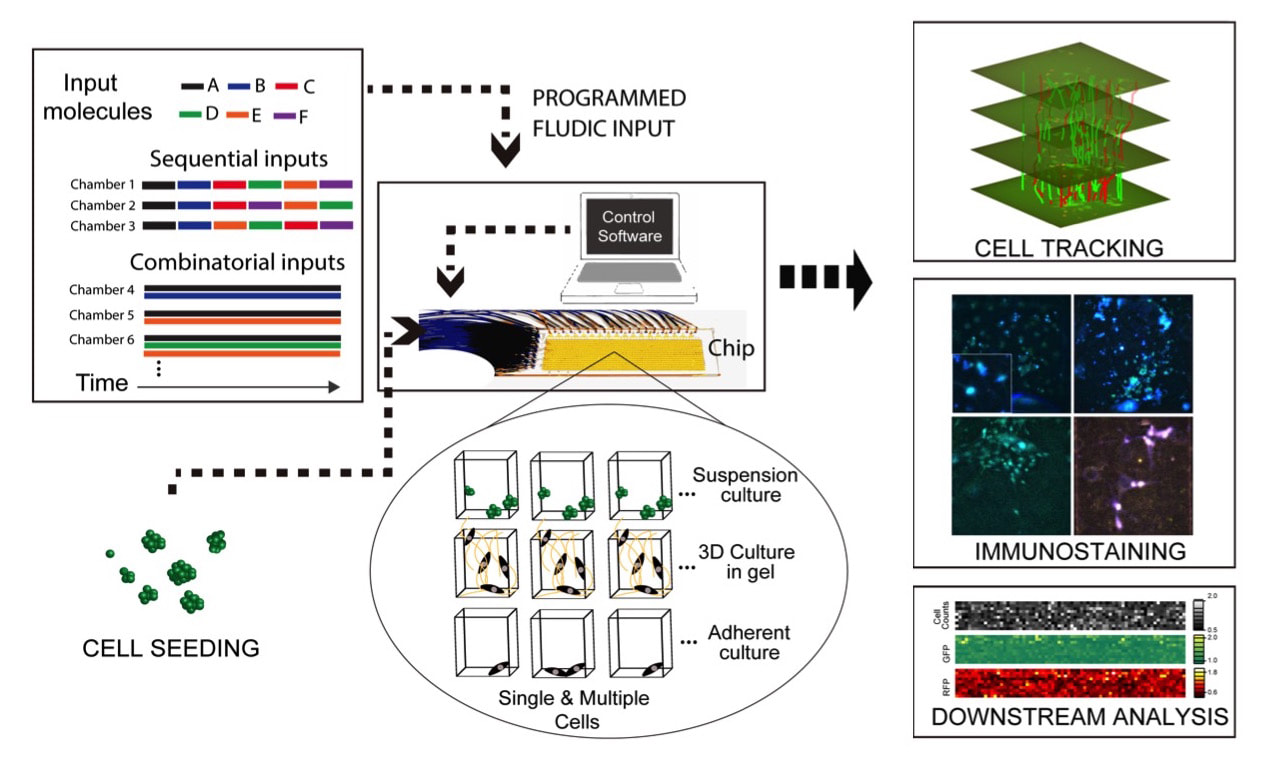
Tay Lab for Bioengineering and Systems BiologyWe want to understand, model and manipulate complex biological systems to help cure diseases. To enable this, we develop high-throughput automated microfluidic and optofluidic systems that perform quantitative, multi-dimensional and dynamic measurements with single-cell resolution.
|
Prof. Savas TaySavas Tay is Professor at the Pritzker School of Molecular Engineering, and the Institute for Genomics and Systems Biology, at The University of Chicago. His scientific goal is to understand "how life works" from an engineer's perspective, and to manipulate biological systems to solve major problems in medicine. Dr. Tay adopts a Systems Biology and Biotechnology approach to biological research, by developing technologies for high-throughput quantitative measurements of single live cells, and by integrating experimental biology and mathematical modeling. More information about Savas Tay is found in his PME website.
|
News
Existing drug masitinib identified as potential inhibitor of SARS-CoV-2, work published on Science: Paper LINK, UChicago News
Dr. Tay received 2019 Allen Distinguished Investigators Award: LINK
New 'lab-on-a-chip' can test thousands of stem cells simultaneously: LINK
UChicago biotech startup raises $2M to develop feces biosensors: LINK
'Lab-on-a-chip' posted on: UChicago News and Phys.org
Getting the scoop on poop: UChicago Wellnews
News Article on GEN
Open Positions: Postdoc Position on spatially resolved single-cell analysis from tissue.
Open Positions: Postdoc Position on developing next generation high-throughput single-cell analysis methods
New paper published on Lab on Chip.
Alumni Dr. Michael Junkin got a position in Complete Genomics.
New paper published on Lab on a Chip.
Read more...
Research Overview
Cell Signaling and Dynamics
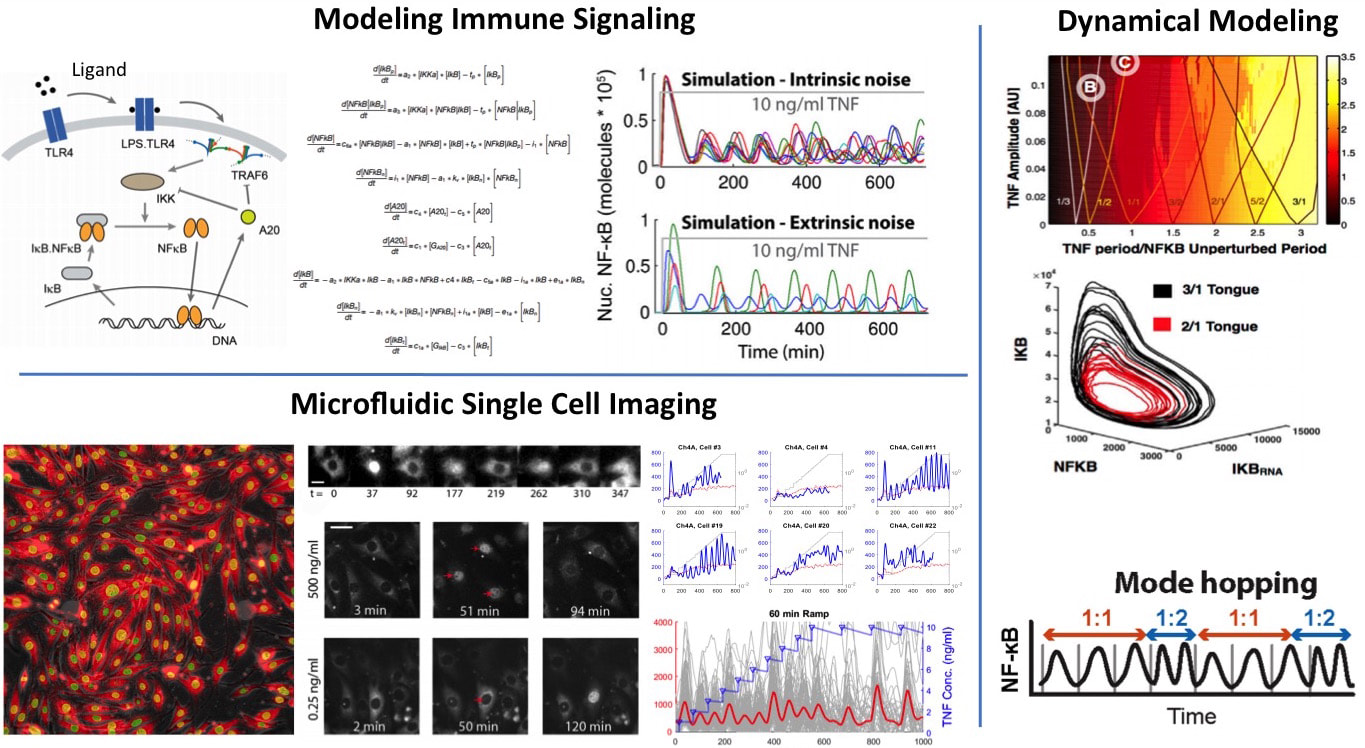
We want to understand how cells process information during immune response. NF-kB is an important innate immune pathway that integrates hundreds of signals including cytokines and pathogens, and controls the expression of thousands of inflammatory genes. We investigated NF-kB dynamics at the single-cell level using high-throughput microfluidic live-cell microscopy and quantitative gene expression (Nature, 2010; eLife 2014; Cell 2015; Cell Systems 2017). We discovered that single-cell responses are highly heterogeneous and are digital, both in cell activation probability and dynamics of the NF-kB transcription factor, as well as downstream gene expression dynamics. To understand the underlying mechanisms and to predict complex signaling scenarios, we developed a stochastic computer model of the NF-kB pathway based on our comprehensive single-cell data. This comprehensive mathematical model reproduces great majority of the observed characteristics under all input signal levels. We are expanding this model and using it as an in silico test-bed for future experiments and discoveries.
Current directions in signaling include the investigation of the mechanisms behind stochastic and digital activation, and the emergent properties of this complex signaling scheme in pathogen-host interactions and tissue inflammation. For this effort, we combine one of the most powerful arsenal of single-cell analytical tools in the world, namely high-throughput automated microfluidic cell culture, automated live cell imaging and tracking, high-throughput single-cell gene expression (qPCR) and digital-PCR, RNA sequencing, single-cell digital protein counting, and single cell multiplexed proteomics. This pipeline, applied to cells expressing fluorescent fusion proteins and reporters, can generate terabytes of dynamic single-cell data every week, with unprecedented degree of control, accuracy and reproducibility. Using these tools and modeling, we are investigating the spatio-temporal emergent properties cell signaling in cell populations and intact tissue. We are trying to understand how NF-kB integrates signals from multiple inputs during virus infection using microfluidic combinatorics and machine learning. We are exploring how environmental and pathway noise influences signal transduction. We discovered that intrinsic noise and cell-to-cell variability actually help NF-kB signaling under dynamical inputs (Cell, 2015). We would like to know if noise in the input also helps signal transduction via stochastic resonance. New technologies based on microfluidics and optogenetics are being developed to test these signaling scenarios.
We want to understand how cells process information during immune response. NF-kB is an important innate immune pathway that integrates hundreds of signals including cytokines and pathogens, and controls the expression of thousands of inflammatory genes. We investigated NF-kB dynamics at the single-cell level using high-throughput microfluidic live-cell microscopy and quantitative gene expression (Nature, 2010; eLife 2014; Cell 2015; Cell Systems 2017). We discovered that single-cell responses are highly heterogeneous and are digital, both in cell activation probability and dynamics of the NF-kB transcription factor, as well as downstream gene expression dynamics. To understand the underlying mechanisms and to predict complex signaling scenarios, we developed a stochastic computer model of the NF-kB pathway based on our comprehensive single-cell data. This comprehensive mathematical model reproduces great majority of the observed characteristics under all input signal levels. We are expanding this model and using it as an in silico test-bed for future experiments and discoveries.
Current directions in signaling include the investigation of the mechanisms behind stochastic and digital activation, and the emergent properties of this complex signaling scheme in pathogen-host interactions and tissue inflammation. For this effort, we combine one of the most powerful arsenal of single-cell analytical tools in the world, namely high-throughput automated microfluidic cell culture, automated live cell imaging and tracking, high-throughput single-cell gene expression (qPCR) and digital-PCR, RNA sequencing, single-cell digital protein counting, and single cell multiplexed proteomics. This pipeline, applied to cells expressing fluorescent fusion proteins and reporters, can generate terabytes of dynamic single-cell data every week, with unprecedented degree of control, accuracy and reproducibility. Using these tools and modeling, we are investigating the spatio-temporal emergent properties cell signaling in cell populations and intact tissue. We are trying to understand how NF-kB integrates signals from multiple inputs during virus infection using microfluidic combinatorics and machine learning. We are exploring how environmental and pathway noise influences signal transduction. We discovered that intrinsic noise and cell-to-cell variability actually help NF-kB signaling under dynamical inputs (Cell, 2015). We would like to know if noise in the input also helps signal transduction via stochastic resonance. New technologies based on microfluidics and optogenetics are being developed to test these signaling scenarios.
Single Cell Proteomics
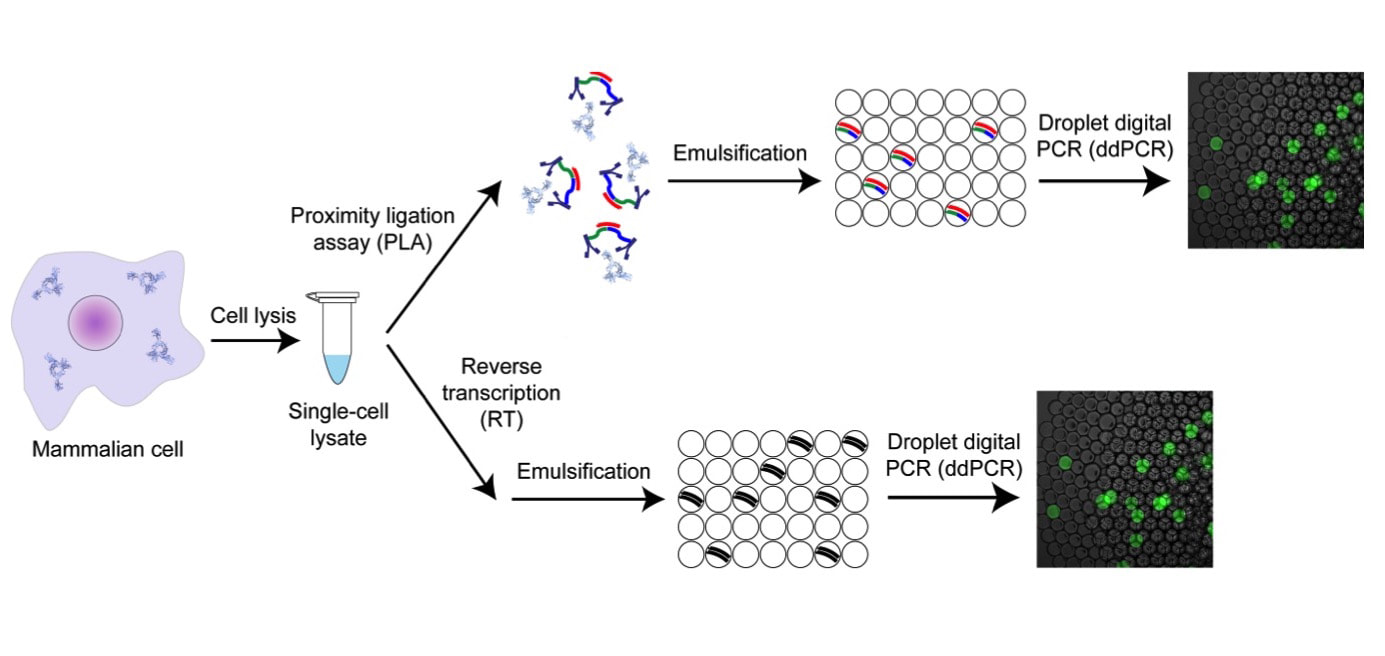
We are developing highly multiplexed single cell proteomic methods. Protein measurements are highly informative on the identity and function of individual cells, however the field is severely lacking in methods with sufficient sensitivity and throughput. We recently developed a method for protein and mRNA counting in individual mammalian cells based on digital PCR and proximity ligation, and used it to mathematically model the central dogma (Molecular Cell, 2016). We are developing a multiplexed version of this method that will allow us to measure thousands of proteins, protein complexes and transcripts in millions of individual cells. We invented a method for spatially resolved proteomic and transcriptomic analysis from intact tissue, where the positions of all the cells in tissue will be recorded and their contents will be measured by RNA sequencing or single cell proteomics. This will allow us to create molecular maps of individual cells in tissue, or a “Cell Atlas” with single cell resolution. We are also applying our digital protein counting method for highly sensitive detection and monitoring of infectious diseases like Sepsis, either in the laboratory or at the Point of Care
We are developing highly multiplexed single cell proteomic methods. Protein measurements are highly informative on the identity and function of individual cells, however the field is severely lacking in methods with sufficient sensitivity and throughput. We recently developed a method for protein and mRNA counting in individual mammalian cells based on digital PCR and proximity ligation, and used it to mathematically model the central dogma (Molecular Cell, 2016). We are developing a multiplexed version of this method that will allow us to measure thousands of proteins, protein complexes and transcripts in millions of individual cells. We invented a method for spatially resolved proteomic and transcriptomic analysis from intact tissue, where the positions of all the cells in tissue will be recorded and their contents will be measured by RNA sequencing or single cell proteomics. This will allow us to create molecular maps of individual cells in tissue, or a “Cell Atlas” with single cell resolution. We are also applying our digital protein counting method for highly sensitive detection and monitoring of infectious diseases like Sepsis, either in the laboratory or at the Point of Care
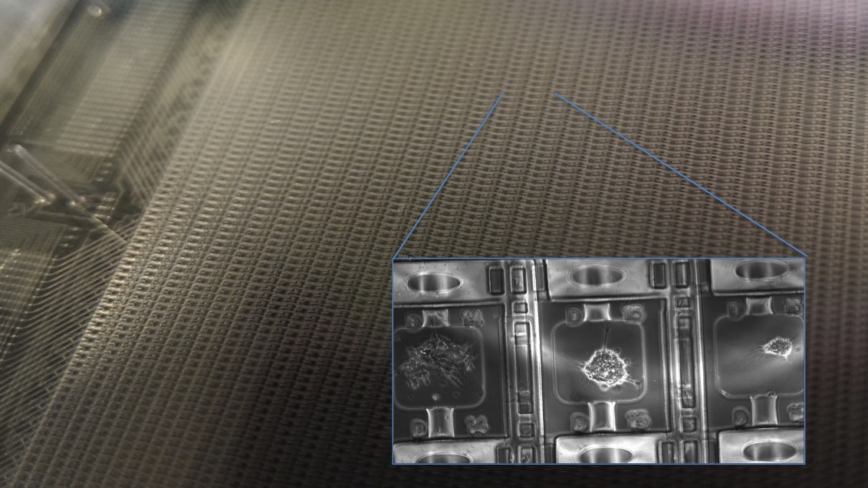
Microfluidic Cell Culture and Dynamical Single Cell Analysis
We developed a range of microfluidic cell culture systems for spatial and temporal analysis of primary cells, from immune cells to neural stem cells. Our cell culture systems can create thousands of dynamical cell cultures in a single experiment. We use these systems and machine learning analysis in high-throughput quantitative mapping of the signaling landscape in immunity and in stem cell differentiation. Our devices can measure secretion of cytokines or other molecules from individual immune cells, in a time dependent manner. We can monitor cell migration in chemokine gradients, and sort individual cells based on their migration characteristics and speed. These systems allow creating cellular environments that mimic infection, immune response, and dynamical cell niches.
We developed a range of microfluidic cell culture systems for spatial and temporal analysis of primary cells, from immune cells to neural stem cells. Our cell culture systems can create thousands of dynamical cell cultures in a single experiment. We use these systems and machine learning analysis in high-throughput quantitative mapping of the signaling landscape in immunity and in stem cell differentiation. Our devices can measure secretion of cytokines or other molecules from individual immune cells, in a time dependent manner. We can monitor cell migration in chemokine gradients, and sort individual cells based on their migration characteristics and speed. These systems allow creating cellular environments that mimic infection, immune response, and dynamical cell niches.
High-Throughput Organoid Culture for Drug Testing
We invented an automated organoid culture device that can create thousands of culture conditions in a single experiment, including drug cocktails. These drugs and signaling molecules can be dynamically changed over time. Our device viably cultures the most demanding organoid types in high throughput fashion. This system is being used for growing primary pancreatic cancer organoids for drug screening in personalized therapy. We collaborate with Kevin White, Andrey Rzhetsky and Tempus in this work. Our devices can culture a range of organoids in various support matrices including matrigel and hydrogels, deliver combinations of drugs to these organoids, change drug conditions when needed, and monitor the growth vs. death of organoids using live-cell microscopy and end-point analysis by sequencing. These will help us develop individualized therapies in cancer.
We invented an automated organoid culture device that can create thousands of culture conditions in a single experiment, including drug cocktails. These drugs and signaling molecules can be dynamically changed over time. Our device viably cultures the most demanding organoid types in high throughput fashion. This system is being used for growing primary pancreatic cancer organoids for drug screening in personalized therapy. We collaborate with Kevin White, Andrey Rzhetsky and Tempus in this work. Our devices can culture a range of organoids in various support matrices including matrigel and hydrogels, deliver combinations of drugs to these organoids, change drug conditions when needed, and monitor the growth vs. death of organoids using live-cell microscopy and end-point analysis by sequencing. These will help us develop individualized therapies in cancer.
Microbiome
We are actively pursuing new directions in microbiome research through our collaborations across the University of Chicago Microbiome Center. We use our microfluidic technologies to sort, cultivate and analyze individual microbes from patients or the environment, and investigate microbe-host interactions through microfluidic co-culture experiments and DNA sequencing.
We are actively pursuing new directions in microbiome research through our collaborations across the University of Chicago Microbiome Center. We use our microfluidic technologies to sort, cultivate and analyze individual microbes from patients or the environment, and investigate microbe-host interactions through microfluidic co-culture experiments and DNA sequencing.
Microfluidic Large-Scale Integration and Automation
Valve-based microfluidics is a robust technology that allows parallelizing and automating everyday laboratory procedures such as mixing, separating, sorting and sampling, in a compact and low cost platform. We use powerful microfluidic cell culture systems for highly multiplexed stimulation and high-throughput time lapse imaging of live cells, creation of spatio-temporal gradients and ligand mixtures. We also use high-throughput microfluidic single-cell gene expression analysis, including digital-PCR, that allow absolute quantitation of mRNA's in a single cell. To further improve our capabilities in systems and cell biology, we will develop new microfluidic tools for investigating cell-cell communication, manipulation and sorting of cells, delivering reagents and genes, and in-vivo detection and quantitating of proteins. We recently developed a new multiplexing method that allows us to control nearly 10,000 individual cell culture chambers in a single device, where each culture can be independently addressed. This is record breaking multiplexing throughput in automated microfluidic culture, and exceeds the state of the art by 100-fold.
Valve-based microfluidics is a robust technology that allows parallelizing and automating everyday laboratory procedures such as mixing, separating, sorting and sampling, in a compact and low cost platform. We use powerful microfluidic cell culture systems for highly multiplexed stimulation and high-throughput time lapse imaging of live cells, creation of spatio-temporal gradients and ligand mixtures. We also use high-throughput microfluidic single-cell gene expression analysis, including digital-PCR, that allow absolute quantitation of mRNA's in a single cell. To further improve our capabilities in systems and cell biology, we will develop new microfluidic tools for investigating cell-cell communication, manipulation and sorting of cells, delivering reagents and genes, and in-vivo detection and quantitating of proteins. We recently developed a new multiplexing method that allows us to control nearly 10,000 individual cell culture chambers in a single device, where each culture can be independently addressed. This is record breaking multiplexing throughput in automated microfluidic culture, and exceeds the state of the art by 100-fold.
Optofluidics
Optics is a powerful tool for characterizing and manipulating biomolecules, cells and tissue. We previously developed methods and technologies based on nonlinear optics and dynamic holography and used them for optical communication and imaging applications. We are combining such optical techniques with microfluidics to realize the full potential of lab-on-a-chip concept in biology and medicine. Technologies we will develop include optofluidic biosensors, integrated hand-held microscopes, new high-throughput microscopy techniques, and photonic/plasmonic systems for imaging and manipulation of cells (sorting, culturing, delivery of drugs or genes). We will also use dynamic holography in photorefractive polymers for imaging of tissue and cells. Photorefractive polymers record dynamic holograms and reconstruct them in real-time, allowing imaging through highly scattering media (i.e. tissue), and three-dimensional imaging and displays.
Optics is a powerful tool for characterizing and manipulating biomolecules, cells and tissue. We previously developed methods and technologies based on nonlinear optics and dynamic holography and used them for optical communication and imaging applications. We are combining such optical techniques with microfluidics to realize the full potential of lab-on-a-chip concept in biology and medicine. Technologies we will develop include optofluidic biosensors, integrated hand-held microscopes, new high-throughput microscopy techniques, and photonic/plasmonic systems for imaging and manipulation of cells (sorting, culturing, delivery of drugs or genes). We will also use dynamic holography in photorefractive polymers for imaging of tissue and cells. Photorefractive polymers record dynamic holograms and reconstruct them in real-time, allowing imaging through highly scattering media (i.e. tissue), and three-dimensional imaging and displays.